Introduction to Organic and Biochemistry, 8th Edition solutions manual and test bank by Frederick A. Bettelheim | William H. Brown
Chapter 2: Atoms
2.1 (a) NaClO3 (b) AlF3
2.2 (a) The mass number is 15 + 16 = 31.
(b) The mass number is 86 + 136 = 222.
2.3 (a) The element has 15 protons, making it phosphorus (P); its symbol is .
(b) The element has 86 protons, making it radon (Rn); its symbol is .
2.4 (a) The atomic number of mercury (Hg) is 80; that of lead (Pb) is 82.
(b) An atom of Hg has 80 protons; an atom of Pb has 82 protons.
(c) The mass number of this isotope of Hg is 80 + 120 = 200; the mass number for this isotope of Pb is 82 + 120 = 202.
(d) The symbols of these isotopes are and
.
2.5 The atomic number of iodine (I) is 53. The number of neutrons in each isotope is 125 - 53 = 72 for iodine-125 and 131 - 53 = 78 for iodine-131. The symbols for these two isotopes are and
.
2.6 The atomic weight is 6.941 amu, which is nearer to 7 amu than 6 amu. Therefore, lithium-7 is the more abundant isotope. The relative abundances for these two isotopes are 92.50 percent for lithium-7 and 7.50 percent for lithium-6.
2.7 This element has 13 electrons and, therefore, 13 protons. The element with atomic number 13 is Aluminum (Al).
2.8 Both Democritus and Dalton believed that matter was composed of tiny indivisible particles referred to as atoms. The major difference between Democritus and Dalton is that Dalton based his theory on evidence rather than belief.
2.9 (b), (c), (d), (f), (g), (h), and (k): True
(a) False: matter is divided into pure substances and mixtures.
(e) False: mixtures can be separated into their component pure substances.
(i) False: technetium, promethium, and all of the elements beyond uranium are man made.
(j) False: H, O, C, N, Ca, and P are the six most important elements in the human body.
(l) False: The combining ratio is based on a ratio of atoms, not a ratio of masses.
2.10 (a) Oxygen - an element (b) Table salt - a compound
(c) Sea water - a mixture (d) Wine - a mixture
(e) Air - a mixture (f) Silver - an element
(g) Diamond - an element (h) A pebble - a mixture
(i) Gasoline - a mixture (j) Milk - a mixture
(k) Carbon dioxide - a compound (l) Bronze - a mixture
2.12 Given here is the element, its symbol, and its atomic number:
(a) Bohrium (Bh, 107) (b) Curium (Cm, 96)
(c) Einsteinium (Es, 99) (d) Fermium (Fm, 100)
(e) Lawrencium (Lr, 103) (f) Meitnerium (Mt, 109)
(g) Mendelevium (Md, 101) (h) Nobelium (No, 102)
(i) Rutherfordium (Rf, 104) (j) Seaborgium (Sg, 106)
2.14 The three elements named for planets are mercury (Hg, 80), uranium (U, 92), and neptunium (Np, 93). Pluto [plutonium (Pu, 94)] was recently demoted from planetary status.
2.16 (a) NaHCO3 (b) C2H6O (c) KMnO4
2.18 The law of conservation of mass
2.20 Mass percent of H and O in:
H2O: 18.015 g/mol H: 11.2% O: 88.8%
H2O2: 34.014 g/mol H: 5.9% O: 94.1%
2.22 (a) Protons are located in the nucleus.
(b) Electrons are outside the nucleus
(c) Neutrons are in the nucleus.
2.24 (a) Mass number = 22 protons + 26 neutrons = 48
(b) Mass number = 76 protons + 114 neutrons = 190
(c) Mass number = 34 protons + 45 neutrons = 79
(d) Mass number = 94 protons + 150 neutrons = 244
2.26 An element is identified by its atomic number, which is mass number - number of neutrons.
(a) 45 - 24 = 21 protons. The element is scandium (Sc), and its symbol is .
(b) 48 - 26 = 22 protons. The element is titanium (Ti), and its symbol is .
(c) 107 - 60 = 47 protons. The element is silver (Ag), and its symbol is .
(d) 246 - 156 = 90 protons. The element is thorium (Th) and its symbol is .
(e) 36 - 18 = 18 protons. The element is argon (Ar) and its symbol is .
2.28 The number of neutrons is equal to the mass number - atomic number (number of protons).
(a) 13 - 6 = 7 neutrons (b) 73 - 32 = 41 neutrons
(c) 188 -76 = 112 neutrons (d) 195 - 78 = 117 neutrons
2.30 (a) Neon-22 has 10 protons and 22 - 10 = 12 neutrons
(b) Palladium-104 has 46 protons and 104 - 46 = 58 neutrons
(c) Chlorine-35 has 17 protons and 35 - 17 = 18 neutrons
(d) Tellurium-128 has 52 protons and 128 - 52 = 76 neutrons
(e) Lithium-7 has 3 protons and 7 - 3 = 4 neutrons
(f) Uranium-238 has 92 protons and 238 - 92 = 146 neutrons
2.32 The atomic number is the number of protons in the nucleus of an element. The mass number is the number of protons and neutrons in the nucleus.
2.34 The atomic weight (121.75 amu) is nearer to that of antimony-121 (120.90 amu) than it is to antimony-123 (122.90 amu). Therefore, antimony-121 has the greater natural abundance. The observed abundances are 57.3 percent antimony-121, and 42.7 percent antimony-123.
2.36 Carbon-14 has 6 protons, 6 electrons, and 8 neutrons.
2.38 Fluorine-18 has 9 protons, 9 electrons, and 9 neutrons.
Nitrogen-13 has 7 protons, 7 electrons, and 6 neutrons.
Oxygen-15 has 8 protons, 8 electrons, and 7 neutrons.
2.40 Rubidium-87 has 37 protons, 37 electrons, and 50 neutrons.
Strontium-87 has 38 protons, 38 electrons, and 49 neutrons.
2.42 In period 3, there are three metals (Na, Mg, and Al), one metalloid (Si) and four nonmetals (P, S, Cl, and Ar).
2.44 Periods 1 - 3 contain more nonmetals than metals. Periods 4 -7 contain more metals than nonmetals.
2.46 Palladium (Pd), cobalt (Co), and chromium (Cr) are transition elements. Cerium (Ce) is an inner transition element; K and Br are main group elements.
2.48 (a) Argon is a nonmetal (b) Boron is a metalloid
(c) Lead is a metal (d) Arsenic is a metalloid
(e) Potassium is a metal (f) Silicon is a metalloid
(g) Iodine is a nonmetal (h) Antimony is a metalloid
(i) Vanadium is a metal (j) Sulfur is a nonmetal
(k) Nitrogen is a nonmetal
2.50 Only period 1 has two elements. Periods 2 and 3 have eight elements. Periods 4 and 5 have 18 elements and period 6 has 32 elements. Period 7 is not yet filled, but it has the potential for 32 elements.
2.52 The group number tells the number of Lewis dots to be placed around the symbol of the element.
2.54 Following are Lewis dot structures for each element in Problem 2.49:
2.56 Following are Lewis dot structures for each element in Problem 2.55:
2.58 In the ground state, 3s and 3p orbitals are occupied by valence electrons.
2.60 (a) Rb(37): 1s22s22p63s23p64s23d104p65s1
(b) Sr(38): 1s22s22p63s23p64s23d104p65s2
(c) Br(35): 1s22s22p63s23p64s23d104p5
2.62 The properties are similar because all of them have the same outer-shell electron configuration. They are not identical because each has a different number of filled inner shells.
2.64 Going from left to right within a period, increasing positive charge holds onto the outer electrons more tightly, thus decreasing the atomic radius and increasing the ionization energy. So, for the following atoms B, C, and N:
(a) Boron has the largest atomic radius.
(b) Nitrogen has the smallest atomic radius.
(c) Nitrogen has the largest ionization energy.
(d) Boron has the lowest ionization energy.
2.66 Ionization energy generally increases from left to right within a period in the Periodic Table and from bottom to top within column:
(a) K < Na < Li (b) C < N < Ne (c) C < O < F (d) Br < Cl < F
2.68 Following are the ground-state electron configurations of Mg atom, Mg+, Mg2+, and Mg3+.
The first electron is removed from the 3s orbital. The removal of each subsequent electron requires more energy because, after the first electron is removed, each subsequent electron is removed from a positive ion, which strongly attracts the remaining electrons. The third ionization energy is especially large because the electron is removed from the filled second principal energy level, meaning that it is removed from an ion that has the same electron configuration as neon.
2.70 The most abundant elements by weight (a) in the Earth's crust are oxygen and silicon, and (b) in the human body they are oxygen and carbon.
2.72 Bronze is an alloy of copper and tin.
2.74 (a) 1s (b) 2s, 2p (c) 3s, 3p, 3d (d) 4s, 4p, 4d, 4f
2.76 (a) The atomic radius decreases going from left to right across a period in the Period Table. Although the principal quantum number of the outermost orbital remains the same, as each successive electron is added, the nuclear charge also increases by the addition of one proton. The resulting increased attraction between nucleus and electrons is somewhat stronger than the increasing repulsion between electrons, which causes the atomic radius to decrease.
(b) To pull a valence electron from an atom, energy is required to overcome the attractive forces on the electron from the positively charged nucleus.
2.78 (a) s2p1 (b) s2p5 (c) s2p3
2.80 (a) Carbon-12 has 12 protons and 12 neutrons. Neutrons contribute 50% of its mass.
(b) Calcium-40 has 20 protons and 20 neutrons. Neutrons contribute 50% of its mass.
(c) Iron-55 has 26 protons and 29 neutrons. Neutrons contribute 53% of its mass.
(d) Bromine-79 has 35 protons and 44 neutrons. Neutrons contribute 56% of its mass.
(e) Platinum-195 has 78 protons and 117 neutrons. Neutrons contribute 60% of its mass.
(f) Uranium-238 has 92 protons and 146 neutrons. Neutrons contribute 61% of its mass.
2.82 (a) P (b) K (c) Na (d) N (e) Br
(f) Ag (g) Ca (h) C (i) Sn (j) Zn
2.84 (a) Silicon is in Group 4A. It has four outer-shell electrons.
(b) Bromine is in Group 7A. It has seven outer-shell electrons.
(c) Phosphorus is in Group 5A. It has five outer-shell electrons.
(d) Potassium is in Group 1A. It has one outer-shell electron.
(e) Helium is in Group 8A. It has two outer-shell electrons.
(f) Calcium is in Group 2A. It has two outer-shell electrons.
(g) Krypton is in Group 8A. It has eight outer-shell electrons.
(h) Lead is in Group 4A. It has four outer-shell electrons.
(i) Selenium is in Group 6A. It has six outer-shell electrons.
(j) Oxygen is in Group 6A. It has six outer-shell electrons.
2.86 (a) An electron has a charge of -1, a proton a charge of +1, and a neutron has no charge.
(b) An electron has a mass of 0.0005 amu; both protons and neutrons have masses of 1 amu.
2.88 Xenon (Xe) will have the highest ionization energy. Ionization energy increases from left to right going across the periodic table.
2.90 Going from left to right across a period in the Periodic Table, protons are being added to the nucleus and electrons are added to the valence shell. For elements in the same period, the principal energy level remains the same (for example, the valence electrons of all second period elements occupy the second principal energy level). But in going from one element to the next across a period, one more proton is added to the nucleus, thus increasing the nuclear charge by one unit for each step from left to right. The result is that the nucleus exerts an increasingly stronger pull on the valence electrons and atomic radius decreases.
2.92 The O2- has a larger radius than F or F- for several reasons. The most important being that O2- has two (-) charged electrons in excess of the positively charged nucleus. This increases the amount of electron-electron repulsions, expanding the electron cloud relative to F and F-. Another factor is that oxygen is less electronegative than fluorine, therefore, oxygen holds on to its electrons less tightly.
2.94 The nucleus takes up only a small portion of the size of an atom. The nucleus also holds most of the mass of the atom. Moving from potassium to vanadium, the atomic radii of the atoms get smaller, but the nuclei gain mass, therefore, the density increases.
2.96 (a) Ti: 1s22s22p63s23p64s23d2
(b) Ti2+: 1s22s22p63s23p64s2
(c) Ti4+: 1s22s22p63s23p6
2.98 The ionization energy is the energy required to remove the most loosely held electron(s) from an atom in the gas phase. The ionization energies of elements decrease going down a group in the Periodic Table. This periodic property occurs because as we go down a group, the valence electrons exist further away from the influence of the positive nucleus, rendering it more easily removed through ionization.
2.100 We can make the following generalizations. As illustrated by the answer to Problem 2.81, the neutron to proton ratio of the elements generally increases as atomic number increases. For light elements (H through Ca), the stable isotopes usually have equal numbers of protons and neutrons. Beyond calcium (Ca), the neutron/proton ratio becomes increasingly greater than 1.
CHAPTER 3 — CHEMICAL BONDS
MULTIPLE CHOICE
1. How many main types of chemical bonds exist?
| a. | 1 | c. | 3 |
| b. | 2 | d. | 4 |
ANS: B PTS: 1
TOP: 3.1 - WHAT DO WE NEED TO KNOW BEFORE WE BEGIN?
2. Which of the following is true in general of all cations?
| a. | A cation has equal numbers of protons and electrons. |
| b. | A cation has fewer protons than electrons. |
| c. | A cation has more protons than electrons. |
| d. | A cation has more protons than neutrons. |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
3. Which of the following is generally true of all anions?
| a. | An anion has equal numbers of protons and electrons. |
| b. | An anion has fewer protons than electrons. |
| c. | An anion has more protons than electrons. |
| d. | An anion has more electrons than neutrons. |
ANS: B PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
4. Which is most likely true for an atom with six valence electrons?
| a. | It will gain one electron. |
| b. | It will gain two electrons. |
| c. | It will lose one electron. |
| d. | It will lose two electrons. |
ANS: B PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
5. Which is most likely true for an atom with one valence electron?
| a. | It will gain one electron. |
| b. | It will gain two electrons. |
| c. | It will lose one electron. |
| d. | It will lose two electrons. |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
6. Which of the following atoms is least likely to form an ion?
| a. | fluorine | c. | neon |
| b. | magnesium | d. | sodium |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
7. For which of the following atoms do we not try to apply the octet rule?
| a. | oxygen | c. | sodium |
| b. | nickel | d. | xenon |
ANS: B PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
8. What is the valence shell electron configuration of all the noble gases except for helium?
| a. | ns2 | c. | ns2np6 |
| b. | ns2np3 | d. | np8 |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
9. Which of the following elements is most likely to lose electrons to become a cation?
| a. | Ar | c. | Cl |
| b. | C | d. | Fe |
ANS: D PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
10. Which of the following elements is most likely to gain electrons to become an anion?
| a. | Ar | c. | Cl |
| b. | C | d. | Fe |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
11. Which of the following occurs when a magnesium atom is converted to Mg2+?
| a. | The magnesium atom gains two electrons and loses two protons. |
| b. | The magnesium atom gains two electrons. |
| c. | The magnesium atom loses two electrons and two protons. |
| d. | The magnesium atom loses two electrons. |
ANS: D PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
12. Which of the following occurs when a sulfur atom is converted to S2–?
| a. | The sulfur atom gains two electrons and loses two protons. |
| b. | The sulfur atom gains two electrons. |
| c. | The sulfur atom loses two electrons and two protons. |
| d. | The sulfur loses two electrons. |
ANS: B PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
13. Which of the following ions has the same electronic configuration as argon?
| a. | K+ | c. | both a and b |
| b. | Ca2+ | d. | neither a nor b |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
14. Which of the following ions has the same electronic configuration as argon?
| a. | K2+ | c. | both a and b |
| b. | Ca+ | d. | neither a nor b |
ANS: D PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
15. In what form is lithium administered when it is used as a drug in the treatment of manic depression?
| a. | Li– | c. | Li+ |
| b. | Li | d. | Li2+ |
ANS: C PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
16. In what form is fluorine administered when it is used to prevent tooth decay?
| a. | F– | c. | F2 |
| b. | F | d. | F+ |
ANS: A PTS: 1 TOP: 3.2 - WHAT IS THE OCTET RULE?
17. What is the name of the species formed when a sodium atom loses an electron?
| a. | sodate | c. | sodium |
| b. | sodide | d. | sodium ion |
ANS: B PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
18. What is the name of the species formed when a bromine atom gains an electron?
| a. | bromate | c. | bromine |
| b. | bromide | d. | bromine ion |
ANS: B PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
19. For which types of elements do we sometimes use the “ous/ic” system in naming ions?
| a. | alkali metals | c. | noble gases |
| b. | halogens | d. | transition metals |
ANS: D PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
20. In the “ous/ic” system which species is named with the “ic” ending?
| a. | the less negative anion | c. | the less positive cation |
| b. | the more negative anion | d. | the more positive cation |
ANS: D PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
21. What is the common name of Cu+?
| a. | copper ion | c. | cupric ion |
| b. | cuprate ion | d. | cuprous ion |
ANS: D PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
22. What is the common name of Cu2+?
| a. | copper ion | c. | cupric ion |
| b. | cuprate ion | d. | cuprous ion |
ANS: C PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
23. Which of the following is the stannic ion?
| a. | Sn+ | c. | Sn3+ |
| b. | Sn2+ | d. | Sn4+ |
ANS: D PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
24. Which of the following is the stannous ion?
| a. | Sn+ | c. | Sn3+ |
| b. | Sn2+ | d. | Sn4+ |
ANS: B PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
25. Which of the following endings is generally associated with a monatomic anion?
| a. | -ade | c. | -ic |
| b. | -ate | d. | -ide |
ANS: D PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
26. If the name of an ion ends in “ate” what type of ion is it most likely to be?
| a. | a monatomic anion | c. | a polyatomic anion |
| b. | a monatomic cation | d. | a polyatomic cation |
ANS: C PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
27. The preferred name for HCO3– is hydrogen carbonate. What is the common name for this ion?
| a. | bicarbonate | c. | bicarbide |
| b. | dicarbonate | d. | dicarbide |
ANS: A PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
28. The “ide” ending is usually, but not always, associated with monatomic anions. Which of the following polyatomic ions has a name which ends in “ide”?
| a. | C2H3O2– | c. | OH– |
| b. | HPO42– | d. | SO42– |
ANS: C PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
29. Which of the following is true of polyatomic ions?
| a. | All are anions. |
| b. | All are cations. |
| c. | The vast majority are anions. |
| d. | The vast majority are cations. |
ANS: C PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
30. What is the relationship between the nitrate ion and the nitrite ion?
| a. | The nitrate ion has one less oxygen atom than does the nitrite ion and both have the same charge. |
| b. | The nitrate ion has one more oxygen atom than does the nitrite ion and both have the same charge. |
| c. | In addition to having one less oxygen atom than nitrite, nitrate also has a larger negative charge. |
| d. | In addition to having one more oxygen atom than nitrite, nitrate also has a larger negative charge. |
ANS: B PTS: 1
TOP: 3.3 - HOW DO WE NAME ANIONS AND CATIONS?
31. An ionic bond is associated with which of the following?
| a. | interactions between nuclei | c. | unequal sharing of electrons |
| b. | equal sharing of electrons | d. | the transfer of electrons |
ANS: D PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
32. A covalent bond is associated with which of the following?
| a. | interactions between nuclei | c. | the transfer of electrons |
| b. | the sharing of electrons | d. | all of these |
ANS: B PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
33. High electronegativities are associated with which type of elements?
| a. | metals | c. | noble gases |
| b. | metalloids | d. | nonmetals |
ANS: D PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
34. Low electronegativities are associated with which type of elements?
| a. | metals | c. | noble gases |
| b. | metalloids | d. | nonmetals |
ANS: A PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
35. Which of the following elements is most electronegative?
| a. | B | c. | N |
| b. | C | d. | O |
ANS: D PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
36. Which halogen atom is most electronegative?
| a. | bromine | c. | fluorine |
| b. | chlorine | d. | iodine |
ANS: C PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
37. Which pair of species is most likely to form an ionic bond?
| a. | two electrically neutral species |
| b. | two electrically charged species, one positive and one negative |
| c. | two negatively charged species |
| d. | two positively charged species |
ANS: B PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
38. Which of the following situations is most likely to result in formation of a covalent bond?
| a. | when an electrically positive species interacts with an electrically negative species |
| b. | when two nonmetallic elements interact to form a compound |
| c. | when two electrically negative species interact |
| d. | when two electrically positive species interact |
ANS: B PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
39. Which of the following statements is generally true about electronegativity?
| a. | Electronegativity decreases as we move left to right and decreases as we move top to bottom. |
| b. | Electronegativity decreases as we move left to right and increases as we move top to bottom. |
| c. | Electronegativity increases as we move left to right and decreases as we move top to bottom. |
| d. | Electronegativity increases as we move left to right and increases as we move top to bottom. |
ANS: C PTS: 1
TOP: 3.4 - WHAT ARE THE TWO MAJOR TYPES OF CHEMICAL BONDS?
40. Which of the following occurs when an ionic bond is formed?
| a. | Electrons are transferred from the more electronegative element to the less electronegative element. |
| b. | Electrons are transferred from the less electronegative element to the more electronegative element. |
| c. | Electrons are shared equally. |
| d. | Electrons are shared unequally. |
ANS: B PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
41. For which of the following pairs are the atoms most likely to form an ionic bond with each other?
| a. | carbon and oxygen | c. | chlorine and oxygen |
| b. | calcium and chlorine | d. | sodium and magnesium |
ANS: B PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
42. Under which of the following conditions is the A—B bond considered to be ionic?
| a. | when A and B have the same electronegativity |
| b. | when the difference between the electronegativities of the atoms is 1.0 |
| c. | when the difference between the electronegativities of the atoms is 1.5 |
| d. | when the difference between the electronegativities of the atoms is 2.0 |
ANS: D PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
43. Which of the following is true of ionic compounds?
| a. | They are liquids at room temperature. |
| b. | They are solids in which individual molecules are present. |
| c. | They are solids in which both cations and anions are present. |
| d. | Depending on the compound any one of the above may apply. |
ANS: C PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
44. What is the formula of the compound formed by potassium and chlorine?
| a. | KCl | c. | KCl3 |
| b. | KCl2 | d. | K2Cl |
ANS: A PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
45. What is the formula of the compound formed by calcium and fluorine?
| a. | CaF | c. | CaF3 |
| b. | CaF2 | d. | Ca2F |
ANS: B PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
46. What is the formula of the compound formed by an iron(III) ion and oxygen?
| a. | FeO | c. | Fe2O3 |
| b. | FeO2 | d. | Fe3O2 |
ANS: C PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
47. What is the formula of the compound formed between barium and oxygen?
| a. | Ba2O2 | c. | BaO2 |
| b. | Ba2O | d. | BaO |
ANS: D PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
48. Which of the following is the correct way to write the formula of the compound formed between the barium ion and the sulfate ion?
| a. | Ba2(SO4)2 | c. | Ba(SO4)2 |
| b. | Ba2SO4 | d. | BaSO4 |
ANS: D PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
49. Which of the following is the correct way to write the formula of the compound formed between the calcium ion and the sulfate ion?
| a. | Ca2(SO4)2 | c. | Ca(SO4)2 |
| b. | Ca2SO4 | d. | CaSO4 |
ANS: D PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
50. What is the formula of the compound formed between the ammonium ion and the carbonate ion?
| a. | NH4CO3 | c. | (NH4)2CO3 |
| b. | NH4(CO3)2 | d. | (NH4)3CO3 |
ANS: C PTS: 1 TOP: 3.5 - WHAT IS AN IONIC BOND?
51. What is the correct name for MgO?
| a. | monomagnesium monoxide | c. | magnesium oxide |
| b. | magnesium monoxide | d. | magnesium oxygen |
ANS: C PTS: 1
TOP: 3.6 - HOW DO WE NAME IONIC COMPOUNDS?
52. What is the correct name for BaI2?
| a. | barium iodine | c. | barium diiodide |
| b. | barium iodide | d. | monobarium diiodide |
ANS: B PTS: 1
TOP: 3.6 - HOW DO WE NAME IONIC COMPOUNDS?
53. What is the correct name for KCl?
| a. | monopotassium monochloride | c. | potassium chloride |
| b. | potassium chlorine | d. | potassium ion chloride ion |
ANS: C PTS: 1
TOP: 3.6 - HOW DO WE NAME IONIC COMPOUNDS?
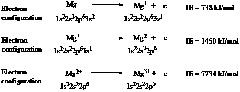
No comments:
Post a Comment