Inquiry into Life by Sylvia Mader and Michael Windelspecht- 14e, solutions manual and test bank test bank 0073525529
Mader - inquiry into life - 14e, solutions manual and test bank 0073525529Inquiry into Life by Sylvia Mader and Michael Windelspecht- 14e, test bank 0073525529
ch2 Key| 1. | If an element has an atomic number of 12, how many electrons are in its outermost shell?
Two electrons fill the innermost shell and eight fill the next, leaving two for the outermost shell. |
| Blooms Level: 3. Apply Learning Outcome: 02.01.01 Describe how protons, neutrons, and electrons relate to atomic structure. Mader - Chapter 02 #1 Section: 02.01 Topic: Chemistry |
| 2. | If an element has an atomic number of 15, then
In an electrically neutral atom, the number of protons (the atomic number) is the same as the number of electrons. The atom would need three orbitals to accommodate 15 electrons, and there would be 5 electrons in its outermost shell. The atomic mass includes the protons and the neutrons; the number of neutrons is not specified in this question. |
| Blooms Level: 3. Apply Learning Outcome: 02.01.02 Use the periodic table to determine relationships among atomic number and mass number. Mader - Chapter 02 #2 Section: 02.01 Topic: Chemistry |
| 3. | The chemical reactivity of an element is dependent on
It is the outermost shell of an atom that can potentially react with electrons in the outermost shells of other atoms. The protons and neutrons remain in the nucleus and do not engage in chemical reactions. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.03 Describe how variations in an atomic nucleus account for its physical properties. Mader - Chapter 02 #3 Section: 02.01 Topic: Chemistry |
| 4. | The atomic mass of an element
The atomic mass is essentially the sum of protons and neutrons in the nucleus; it is not changed by chemical reactions. The mass of electrons is negligible. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.02 Use the periodic table to determine relationships among atomic number and mass number. Mader - Chapter 02 #4 Section: 02.01 Topic: Chemistry |
| 5. | The nucleus of an atom contains
The nucleus contains protons and neutrons; electrons orbit the nucleus. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.01 Describe how protons, neutrons, and electrons relate to atomic structure. Mader - Chapter 02 #5 Section: 02.01 Topic: Chemistry |
| 6. | Isotopes of a given element have
Isotopes are atoms of the same element that differ in the number of neutrons only; thus, they have the same atomic number but different atomic masses. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.02 Use the periodic table to determine relationships among atomic number and mass number. Mader - Chapter 02 #6 Section: 02.01 Topic: Chemistry |
| 7. | The isotope 146C has
Carbon 14 has eight neutrons, 6 protons (and thus 6 electrons), and an atomic mass of 14. |
| Blooms Level: 2. Understand Learning Outcome: 02.01.03 Describe how variations in an atomic nucleus account for its physical properties. Mader - Chapter 02 #7 Section: 02.01 Topic: Chemistry |
| 8. | To measure the activity of the human brain during certain thought processes, a short-lived radioactive sugar is injected in the carotid artery and is utilized by those cells that are most active. This shows up on a PET scan and demonstrates the detection of
The isotopes used in PET scans constitute a low level of radiation used for beneficial purposes. Types of chemical bonds are not registered by this procedure. Although isotopes of a given element differ in their numbers of neutrons, neutrons are not detected by PET. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.04 Identify the beneficial and harmful uses of radiation. Mader - Chapter 02 #8 Section: 02.01 Topic: Chemistry |
| 9. | The difference between 126C and 146C is
Carbon 12 and carbon 14 are different isotopes of the element carbon; this means they have the same number of protons and electrons, but they differ in the number of neutrons. The number of bonds an isotope can form is not determined by its neutrons. |
| Blooms Level: 1. Remember Learning Outcome: 02.01.03 Describe how variations in an atomic nucleus account for its physical properties. Mader - Chapter 02 #9 Section: 02.01 Topic: Chemistry |
| 10. | Which of the following molecules is NOT a compound?
A compound consists of atoms of two or more different elements bound together; H2 is two molecules of the same element (hydrogen) bound together. |
| Blooms Level: 2. Understand Learning Outcome: 02.02.01 Describe how elements are combined into molecules and compounds. Mader - Chapter 02 #10 Section: 02.02 Topic: Chemistry |
| 11. | Which of the following statements is NOT true of chemical bonds?
Salts, such as sodium chloride (NaCl) are ionic compounds. All the other answer choices are accurate statements. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #11 Section: 02.02 Topic: Chemistry |
| 12. | An ion is an atom that
An ion has more or less electrons than a neutral atom of the same element, so it has a net positive or negative charge. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #12 Section: 02.02 Topic: Chemistry |
| 13. | If neutral atoms become positive ions, they
The electron transfer is what will determine if an atom becomes a positive or negative ion. To become a positive ion an atom will need to lose electrons so there are more protons than electrons. |
| Blooms Level: 2. Understand Learning Outcome: 02.02.01 Describe how elements are combined into molecules and compounds. Mader - Chapter 02 #13 Section: 02.02 Topic: Chemistry |
| 14. | When an ionic bond forms, electrons are
Ionic compounds form when one atom gives up an electron, which is accepted by the other member of the pair. Now that one atom is a positively-charged ion and the other is a negatively-charged ion, the two atoms are attracted to one another. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #14 Section: 02.02 Topic: Chemistry |
| 15. | Calcium chloride, CaCl2, is an ionic compound in which
In the ionic compound CaCl2, calcium has transferred two electrons from its outermost shell, becoming a calcium ion (Ca2+). One of the electrons has been accepted by each chlorine atom, so they become chloride ions (Cl-). |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #15 Section: 02.02 Topic: Chemistry |
| 16. | A covalent bond is
A covalent bond results when two atoms share electrons in such a way that each atom has eight electrons in its outermost shell. In contrast, ionic compounds result from the complete transfer of electrons between bonded atoms. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #16 Section: 02.02 Topic: Chemistry |
| 17. | Potassium, a metal with one electron in the outermost shell, will react with how many chlorine atoms? (Chlorine is a nonmetal with seven electrons in the outermost shell.)
Potassium will attain stability by transferring the one electron in its outermost shell to chlorine, which needs only one more electron in its outermost shell to become stable. The result will be a potassium ion (K+) and a chloride ion (Cl-). The two oppositely-charged ions will be attracted to one another, thus forming an ionic compound. |
| Blooms Level: 2. Understand Learning Outcome: 02.02.01 Describe how elements are combined into molecules and compounds. Mader - Chapter 02 #17 Section: 02.02 Topic: Chemistry |
| 18. | Polar covalent bonds result from
Covalent bonds result from sharing of electrons between bound atoms; when the sharing is unequal, it is a polar bond, and when the sharing is equal, it is a nonpolar bond. Ionic bonds are a different type of chemical bond from covalent bonds; in an ionic bond, one or more electrons are completely transferred from one member of the compound to the other(s). Hydrogen bonding is a relatively weak attraction between hydrogen in one molecule and a highly electronegative atom (such as O or N) in an adjacent molecule. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #18 Section: 02.02 Topic: Chemistry |
| 19. | Which of the following statements about hydrogen bonding is incorrect?
Hydrogen bonding is not limited to bonding between adjacent water molecules. For example, hydrogen bonds can form between hydrogen in water and nitrogen in ammonia, or between the two strands of a DNA molecule. Hydrogen bonds are important in determining the shape of large, complex molecules such as proteins. Even so, hydrogen bonds are relatively weak when compared to ionic or covalent bonds, and are easily broken. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. Mader - Chapter 02 #19 Section: 02.02 Topic: Chemistry |
| 20. | Which of the following is not a property of water that results from hydrogen bonding?
Due to its hydrogen bonding, water melts at 0°C instead of -100°C. All the other answer choices are accurate statements. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #20 Section: 02.03 Topic: Chemistry |
| 21. | Water is a liquid at room temperature. This is due to
Hydrogen bonding between water molecules keeps water in a liquid state at temperatures typically found on the Earth's surface, including room temperature. Water molecules do not covalently bond to one another, and the water molecule is too small to permit intramolecular hydrogen bonds to form. Water molecules do ionize, but this does not influence the fluid nature of water. |
| Blooms Level: 3. Apply Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #21 Section: 02.03 Topic: Chemistry |
| 22. | The moon lacks life and varies dramatically in temperature. If we could keep a layer of water spread on the surface of the moon, what effect would it have?
Water has a high heat capacity; this serves to moderate temperature changes. Although water does have a high heat of vaporization, this also has a moderating effect, and would prevent temperatures from rising to the highest extremes. |
| Blooms Level: 4. Analyze Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #22 Section: 02.03 Topic: Chemistry |
| 23. | In water, a weak hydrogen bond occurs between hydrogen in one molecule and
Because water molecules are polar, and each oxygen has slight negative charge and the hydrogen a slight positive charge, hydrogen bonding occurs between a hydrogen atom of one water molecule and the oxygen of another. |
| Blooms Level: 1. Remember Learning Outcome: 02.02.03 Compare the relative strengths of ionic, covalent, and hydrogen bonds. Mader - Chapter 02 #23 Section: 02.02 Topic: Chemistry |
| 24. | You notice that rain water forms "beads" on your car. This is an example of what property of water?
The formation of water beads on the surface of a car is due to the cohesiveness of water molecules for one another, thanks to hydrogen bonding. Adhesion is attraction of water molecules for a surface--a property that is not demonstrated here, since the surface of the car (especially a freshly-waxed car) repels the water. The high heat of vaporization and solvent capabilities of water are not apparent in this example. Water does dissociate into ions, but this does not manifest in the formation of beads. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #24 Section: 02.03 Topic: Chemistry |
| 25. | Hydrogen bonding produces which following property of water?
Due to the increased stability of hydrogen bonding at lower temperatures, water is less dense as ice than as liquid water. Water can absorb and release heat, but with a relatively small change in temperature. Due to hydrogen bonding, water boils at 100°C; without hydrogen bonding, it would boil at -91°C. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #25 Section: 02.03 Topic: Chemistry |
| 26. | The water strider is an insect that skates across the water without sinking. The tips of its feet must be coated with molecules that are
The water strider's feet should be coated with hydrophobic molecules to repel water; a hydrophilic coating would cause the insect to stick to the surface of the water and sink. Ions, acids and bases are hydrophilic. |
| Blooms Level: 3. Apply Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. Mader - Chapter 02 #26 Section: 02.03 Topic: Chemistry |
| 27. | The lower the pH
The pH scale is based on hydrogen ion (H+) concentration. The higher the concentration of H+ (and the lower the concentration of OH-, hydroxide), the lower the pH, and the more acidic the solution. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #27 Section: 02.03 Topic: Chemistry |
| 28. | Since pure water is neutral, it contains
Pure water is neutral, with a pH of 7 (midway between 0 and 14 on the pH scale), meaning that it has equal concentrations of hydrogen ions (H+) and hydroxide ions (OH-). |
| Blooms Level: 2. Understand Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #28 Section: 02.03 Topic: Chemistry |
| 29. | The pH of blood is slightly basic. Which of the following would therefore be an expected pH for blood?
The pH scale ranges from 0 to 14, with 7 being neutral. Numbers lower than 7 are acidic, and those higher than 7 are basic. Thus, a pH of 7.4 would be slightly basic. |
| Blooms Level: 3. Apply Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #29 Section: 02.03 Topic: Chemistry |
| 30. | Potassium hydroxide (KOH) almost completely dissociates in aqueous solution into K+ and OH- and is therefore a
Because potassium hydroxide dissociates completely and adds hydroxide ions (OH-) to an aqueous solution, it is a strong base. A weak base would not dissociate so completely. An acid would contribute hydrogen ions (H+) to an aqueous solution. Potassium hydroxide is an ionic compound; a nonpolar covalent molecule would not dissociate or dissolve in an aqueous solution. |
| Blooms Level: 4. Analyze Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #30 Section: 02.03 Topic: Chemistry |
| 31. | Which statement regarding acids and bases is correct?
Acids raise the hydrogen ion (H+) content of a solution, while bases reduce the proportion of H+. The lower the pH, the more acidic the solution, and the higher the pH, the more basic the solution. Strong acids and bases are both harmful. When acids combine with bases, salts result. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #31 Section: 02.03 Topic: Chemistry |
| 32. | Buffers
Buffers are the chemicals or combinations of chemicals that keep pH within normal limits. Weak acids and bases may be used as buffers, not strong ones, which would greatly influence the H+ concentration of the solution and thereby raise or lower the pH. |
| Blooms Level: 1. Remember Learning Outcome: 02.03.03 Describe how buffers are important to living organisms. Mader - Chapter 02 #32 Section: 02.03 Topic: Chemistry |
| 33. | Aspirin is acetyl salicylic acid and can therefore pose a problem to people who have ulcers. Bufferin uses a buffer to neutralize this effect by
Bufferin contains a buffering system to bind the excess H+ from the aspirin (acetyl salicylic acid). This would not be a salt; salts form when acids and bases react. |
| Blooms Level: 3. Apply Learning Outcome: 02.03.03 Describe how buffers are important to living organisms. Mader - Chapter 02 #33 Section: 02.03 Topic: Chemistry |
| 34. | Rain falling in the northeastern U.S. has a pH between 5.0 and 4.0. Normally, rainwater has a pH of about 5.6. Which of the following statements is not correct?
The pH of rainwater is normally acidic (5.6) not neutral (7). All the other answer choices are accurate statements. |
| Blooms Level: 2. Understand Learning Outcome: 02.03.02 Identify common acidic and basic substances. Mader - Chapter 02 #34 Section: 02.03 Topic: Chemistry |
| 35. | Organic molecules
Organic molecules, by definition, must contain both carbon and hydrogen. They are found in organisms and in food, but can also be made in a laboratory. |
| Blooms Level: 1. Remember Learning Outcome: 02.04.01 Compare inorganic molecules to organic molecules. Mader - Chapter 02 #35 Section: 02.04 Topic: Chemistry |
| 36. | Which of the following molecules is inorganic?
Carbon dioxide (CO2) is inorganic because, although it contains carbon, it does not contain hydrogen. All the other molecules contain both carbon and hydrogen and are therefore organic. |
| Blooms Level: 2. Understand Learning Outcome: 02.04.01 Compare inorganic molecules to organic molecules. Mader - Chapter 02 #36 Section: 02.04 Topic: Chemistry |
| 37. | One carbon atom can form covalent bonds with up to ___ other atoms to form an organic molecule.
Carbon, with an atomic number of 6, has 4 electrons in its outermost shell. Thus, carbon can form 4 single covalent bonds with other atoms. |
| Blooms Level: 1. Remember Learning Outcome: 02.04.01 Compare inorganic molecules to organic molecules. Mader - Chapter 02 #37 Section: 02.04 Topic: Chemistry |
| 38. | Two molecules of glucose combine to form a disaccharide molecule during a(n) ________ reaction.
The glucose molecules are monomers; forming a covalent bond between them requires a dehydration reaction. A hydrolysis reaction could be used to break the disaccharide apart into individual glucose monomers. An inert material would not react at all. |
| Blooms Level: 1. Remember Learning Outcome: 02.04.03 Recognize how monomers are joined to form polymers. Mader - Chapter 02 #38 Section: 02.04 Topic: Chemistry |
| 39. | DNA codes for the sequence of amino acids in the primary structure of a protein, but not for sugars or lipids. This is because
Proteins are the main structural and functional components of cells. As enzymes, proteins catalyze the synthesis and degradation of other biological molecules, including sugars and lipids. |
| Blooms Level: 3. Apply Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #39 Section: 02.07 Topic: Chemistry |
| 40. | A genetic mutation can cause a change in the sequence of the 20 amino acids used to build proteins. Such a change is a change to the protein's
A mutation (a change in a DNA sequence) may directly alter the primary structure of a protein, since this is the sequence of amino acids in the chain. However, the primary level of structure dictates the higher levels of structure--secondary, tertiary, and even quaternary--so these may be indirectly affected as a result of the mutation. |
| Blooms Level: 3. Apply Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #40 Section: 02.07 Topic: Chemistry |
| 41. | Glycogen is a
Glycogen (a polysaccharide) is the storage form of glucose (a monosaccharide), which is rich in chemical bond energy, found in animal tissues such as liver and skeletal muscle. DNA would be a nucleic acid found in the nucleus of a cell, and trans fats are found in margarine. |
| Blooms Level: 1. Remember Learning Outcome: 02.05.02 List several examples of important monosaccharides and polysaccharides. Mader - Chapter 02 #41 Section: 02.05 Topic: Chemistry |
| 42. | Maltose is classified as a
Maltose is classified as a carbohydrate due to its carbon and hydrogen backbone. |
| Blooms Level: 1. Remember Learning Outcome: 02.05.02 List several examples of important monosaccharides and polysaccharides. Mader - Chapter 02 #42 Section: 02.05 Topic: Chemistry |
| 43. | All carbohydrate molecules
Carbohydrates are organic molecules, thus, they contain both carbon and hydrogen atoms. They are further characterized by the hydroxyl (-OH) functional group. Although some carbohydrates do contain nitrogen, this is not a requirement in order to be classified as a carbohydrate. Amino acids are the monomers of proteins, not carbohydrates. Carbohydrates do not release H+ in aqueous solutions, so they are not organic acids. |
| Blooms Level: 2. Understand Learning Outcome: 02.05.01 Identify the structural components of a carbohydrate. Mader - Chapter 02 #43 Section: 02.05 Topic: Chemistry |
| 44. | Two glucose molecules can combine to form a disaccharide molecule and
Glucose is a monosaccharide (a carbohydrate monomer); two glucose molecules combine to form a disaccharide, not a dipeptide or a lipid. In the process, a water molecule is released, which is why this is called a dehydration reaction. |
| Blooms Level: 1. Remember Learning Outcome: 02.04.03 Recognize how monomers are joined to form polymers. Learning Outcome: 02.05.01 Identify the structural components of a carbohydrate. Mader - Chapter 02 #44 Section: 02.04 Section: 02.05 Topic: Chemistry |
| 45. | ____ is a polysaccharide that is found in plant cell walls and accounts for their strength.
Cellulose is a structural polysaccharide found in plant cell walls. Starch and glycogen are storage forms of glucose found in plants and animals, respectively. Chitin is a polysaccharide found in the cell walls of fungi and the exoskeletons of arthropods. DNA is a nucleic acid, not a polysaccharide, and is found in cell nuclei. |
| Blooms Level: 1. Remember Learning Outcome: 02.05.02 List several examples of important monosaccharides and polysaccharides. Mader - Chapter 02 #45 Section: 02.05 Topic: Chemistry |
| 46. | Hydrolysis of a fat results in the formation of
A triglyceride molecule (commonly known as a fat) is composed of three fatty acids covalently bonded to glycerol. No monosaccharides, including glucose, make up a triglyceride. |
| Blooms Level: 2. Understand Learning Outcome: 02.06.01 Compare the structures of fats, phospholipids, and steroids. Mader - Chapter 02 #46 Section: 02.06 Topic: Chemistry |
| 47. | A long chain of carbon atoms with hydrogen atoms attached, ending in the acidic group -COOH would be a(n)
A fatty acid is a hydrocarbon chain ending with a -COOH group, which is acidic; a triglyceride is composed of three fatty acids bound to glycerol. Amino acids are the monomers of proteins, and monosaccharides are carbohydrates. Nucleic acids are polymers of nucleotides such as DNA and RNA. |
| Blooms Level: 1. Remember Learning Outcome: 02.06.01 Compare the structures of fats, phospholipids, and steroids. Mader - Chapter 02 #47 Section: 02.06 Topic: Chemistry |
| 48. | Nucleic acids are polymers of
Nucleic acids such as DNA and RNA are polymers of nucleotides. Polysaccharides are polymers of monosaccharides, triglycerides are made up of fatty acids bound to glycerol, and proteins are composed of amino acids. |
| Blooms Level: 1. Remember Learning Outcome: 02.08.01 Compare the structure and function of DNA and RNA. Mader - Chapter 02 #48 Section: 02.08 Topic: Chemistry |
| Blooms Level: 1. Remember Figure: 02.24 Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #49 Section: 02.07 Topic: Chemistry |
| 50. | Enzymes are organic compounds classified as
Enzymes are proteins that speed chemical reactions in living things. Their function is very dependent upon their structure. Steroids and other lipids do not function as enzymes, nor do carbohydrates. With a very few exceptions, neither do nucleic acids. |
| Blooms Level: 1. Remember Learning Outcome: 02.07.01 Describe the functions of proteins in cells. Mader - Chapter 02 #50 Section: 02.07 Topic: Chemistry |
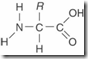
| 51. | The _____ structure of a protein consists of the sequence of the amino acids joined together by peptide bonds.
Amino acids joined together by peptide bonds constitute the primary level of protein structure. In secondary structure, hydrogen bonding between amino acids causes the polypeptide to form an alpha helix or a beta pleated sheet. In tertiary structure, interactions such as covalent bonds between R groups cause the polypeptide to fold and twist. When two or more polypeptides join together, this represents a quaternary level of structure. There is no level of protein structure termed the molecular level. |
| Blooms Level: 1. Remember Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #51 Section: 02.07 Topic: Chemistry |
| 52. | Hemoglobin is a protein composed of two pairs of polypeptide chains. What is the highest level of protein structure represented in hemoglobin?
Amino acids joined together by peptide bonds constitute the primary level of protein structure. In secondary structure, hydrogen bonding between amino acids causes the polypeptide to form an alpha helix or a beta pleated sheet. In tertiary structure, interactions such as covalent bonds between R groups cause the polypeptide to fold and twist. When two or more polypeptides join together, this represents a quaternary level of structure. There is no level of protein structure termed the molecular level. |
| Blooms Level: 2. Understand Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #52 Section: 02.07 Topic: Chemistry |
| 53. | The proposed cause of CJD and kuru in humans, mad cow disease, and scrapie in sheep is a change in a brain protein. Disease victims appear to have a protein that should normally contain alpha helices but instead they have changed into a protein made of beta pleated sheets. The disease appears to spread when the abnormal protein comes into contact with the normal protein, causing it to become deformed. Which level of protein structure is associated with these diseases?
Amino acids joined together by peptide bonds constitute the primary level of protein structure. In secondary structure, hydrogen bonding between amino acids causes the polypeptide to form an alpha helix or a beta pleated sheet--this is the level affected in CJD and similar brain diseases. In tertiary structure, interactions such as covalent bonds between R groups cause the polypeptide to fold and twist. When two or more polypeptides join together, this represents a quaternary level of structure. There is no level of protein structure termed the molecular level. |
| Blooms Level: 3. Apply Learning Outcome: 02.07.01 Describe the functions of proteins in cells. Learning Outcome: 02.07.03 Compare the four levels of protein structure. Mader - Chapter 02 #53 Section: 02.07 Topic: Chemistry |
| 54. | Which of these combinations would be found in a nucleotide?
A nucleotide is composed of a nitrogenous base, a pentose sugar, and a phosphate group. Adenine, thymine, and uracil are bases. Combining an acid and a base yields a salt. Sugars, proteins, and fats are all biological organic molecules. DNA and RNA are nucleic acids composed of nucleotides. Both are made in the nucleus of a cell. |
| Blooms Level: 2. Understand Learning Outcome: 02.08.01 Compare the structure and function of DNA and RNA. Mader - Chapter 02 #54 Section: 02.08 Topic: Chemistry |
| 55. | The backbone of a nucleic acid strand is composed of
The backbone of a nucleic acid such as DNA is composed of alternating pentose sugars and phosphate groups. Glycerol is the backbone of a triglyceride. Although nitrogenous bases such as adenine and thymine are part of DNA, they do not make up the backbone. R groups are part of amino acids, not nucleotides. |
| Blooms Level: 1. Remember Learning Outcome: 02.08.01 Compare the structure and function of DNA and RNA. Mader - Chapter 02 #55 Section: 02.08 Topic: Chemistry |
| 56. | In the search to discover the agents that cause mad cow disease, scrapie in sheep, and CJD and kuru in humans, diseased brain tissues were passed through a fine filter to remove bacteria. The filtrate was still infectious, indicating that something smaller than bacteria, either viruses or organic molecules, must be the causative agent. If a virus were responsible for these brain diseases, then the infectious agent would contain either RNA or DNA. Other possibilities were that the agent was a carbohydrate, fat, or protein. Tissue filtrates were treated with agents that destroyed just one of these chemicals and then injected into a healthy animal, with the results as follows. What is the infectious agent? •Amylase digests carbohydrates; tissue filtrate still infects healthy test animal. •Lipase digests fats; tissue filtrate still infects healthy test animal. •Formaldehyde and/or heat denatures DNA and RNA; tissue filtrate still infects healthy test animal. •Trypsin digests protein; tissue filtrate does not infect healthy test animal.
CJD, kuru, mad cow disease, and scrapie are caused by prions--infectious proteins. This was supported by the finding that only trypsin, which digests proteins, was able to deactivate the infectious agent. |
| Blooms Level: 5. Evaluate Learning Outcome: 02.07.01 Describe the functions of proteins in cells. Mader - Chapter 02 #56 Section: 02.07 Topic: Chemistry |
| 57. | The final shape of a protein is very important to its function. When proteins undergo an irreversible change in shape called ________________ they ________________ perform their usual functions.
Denaturation is when a protein loses its shape and cannot function. Although dehydration reactions do join amino acids together, this represents only the most basic (primary) level of protein structure. |
| Blooms Level: 1. Remember Learning Outcome: 02.07.02 Explain how a polypeptide is constructed from amino acids. Mader - Chapter 02 #57 Section: 02.07 Topic: Chemistry |
| 58. | The primary function of carbohydrates is
Carbohydrates are primarily fuel and short-term energy storage molecules, although some polysaccharides do reinforce cell walls in certain organisms. DNA, a nucleic acid, encodes hereditary information. Proteins can function as enzymes to speed chemical reactions, or as transporters to move molecules across cell membranes. |
| Blooms Level: 1. Remember Learning Outcome: 02.05.02 List several examples of important monosaccharides and polysaccharides. Mader - Chapter 02 #58 Section: 02.05 Topic: Chemistry |
| 59. | Which of the following types of lipid is the most abundant constituent of cell membranes?
Phospholipids are the most abudant type of lipid in cell membranes. Animal cells also have cholesterol in their membranes, but it is less abundant than phospholipid. Triglycerides, also known as neutral fats or simply fats, are energy-storage molecules, not structural molecules. |
| Blooms Level: 1. Remember Learning Outcome: 02.06.02 Identify the functions lipids play in our bodies. Mader - Chapter 02 #59 Section: 02.06 Topic: Chemistry |
| 60. | Which type of lipid molecule is characterized by a backbone of four fused rings?
Only steroids are characterized by their backbone of four fused rings. Phospholipids and triglycerides are lipids, but they do not share the same structure as steroids. Amino acids and DNA are not lipids. |
| Blooms Level: 1. Remember Learning Outcome: 02.06.01 Compare the structures of fats, phospholipids, and steroids. Mader - Chapter 02 #60 Section: 02.06 Topic: Chemistry |
| 61. | Which statement about the cellular nucleic acids DNA and RNA is incorrect?
In RNA, the base uracil replaces thymine. All the other answer choices are accurate statements. |
| Blooms Level: 2. Understand Learning Outcome: 02.08.01 Compare the structure and function of DNA and RNA. Mader - Chapter 02 #61 Section: 02.08 Topic: Chemistry |
| 62. | Which of the following radioactive isotopes are used to detect whether or not an individual has a healthy thyroid?
I131 is a radioactive isotope that is used to detect whether the thyroid is healthy or not. C14 is used to date the age of fossils. Glucose is not taken up by the thyroid. H2 is a gas commonly found in the atmosphere. |
| Blooms Level: 4. Analyze Learning Outcome: 02.01.04 Identify the beneficial and harmful uses of radiation. Mader - Chapter 02 #62 Section: 02.01 Topic: Chemistry |
| 63. | Which of the following subatomic particles will be found within the nucleus of the atom?
The nucleus of an atom will contain the protons & neutrons. Electrons are found in the electron orbitals which circle the nucleus. |
| Blooms Level: 2. Understand Learning Outcome: 02.01.01 Describe how protons, neutrons, and electrons relate to atomic structure. Mader - Chapter 02 #63 Section: 02.01 Topic: Chemistry |
| 64. | Which of the following radiation uses is the one that is most likely to have beneficial and harmful consequences?
When using radiation on a person for cancer treatment there is the possibility of destroying healthy cells along with the cancer cells. Sterilizing mail, surgical equipment and fruits and vegetables tends not to have any type of consequence on people. |
| Blooms Level: 4. Analyze Learning Outcome: 02.01.04 Identify the beneficial and harmful uses of radiation. Mader - Chapter 02 #64 Section: 02.01 Topic: Chemistry |
| 65. | Which of the following sequences correctly lists the bonds in order of strongest to weakest?
The strongest bond listed here is the double covalent followed by the single covalent, ionic and then hydrogen bonds. |
| Blooms Level: 4. Analyze Learning Outcome: 02.02.03 Compare the relative strengths of ionic, covalent, and hydrogen bonds. Mader - Chapter 02 #65 Section: 02.02 Topic: Chemistry |
| 66. | Which type of bond formation is responsible for the properties of water?
Hydrogen bonds are the attraction between the hydrogen of one water molecule and the oxygen of a second water molecule. This attraction sets up the properties of water. A polar covalent bond forms between the hydrogen and oxygen of a particular water molecule. Water doesn't form ionic or nonpolar covalent bonds. |
| Blooms Level: 2. Understand Learning Outcome: 02.02.03 Compare the relative strengths of ionic, covalent, and hydrogen bonds. Mader - Chapter 02 #66 Section: 02.02 Topic: Chemistry |
| 67. | Which functional groups are associated with a dehydration reaction?
H and OH are associated with the dehydration reactions. |
| Blooms Level: 2. Understand Learning Outcome: 02.04.02 Identify the role of a functional group. Mader - Chapter 02 #67 Section: 02.04 Topic: Chemistry |
| 68. | Which of the following functional groups is present in amino acids?
All of these functional groups are present in amino acids. |
| Blooms Level: 2. Understand Learning Outcome: 02.04.02 Identify the role of a functional group. Mader - Chapter 02 #68 Section: 02.04 Topic: Chemistry |
| 69. | Removal of the hydroxyl functional group would disrupt the structure of _________.
Hydroxyl is a functional group found within both sugars and some amino acids. If it was removed it would disrupt their structure. |
| Blooms Level: 4. Analyze Learning Outcome: 02.04.02 Identify the role of a functional group. Mader - Chapter 02 #69 Section: 02.04 Topic: Chemistry |
| 70. | Which of the following reactions is most likely to occur if an individual was to ingest a large dose of lemon juice?
Lemon juice is acidic so it contains large amounts H+. When H+enters the bloodstream it combines with HCO3- to form H2CO3 (carbonic acid). The carbonic acid prevents the blood pH from shifting toward a more acidic or basic range. If it didn't form, in this case the individual's blood would become more acidic and shift toward 7.2. |
| Blooms Level: 5. Evaluate Learning Outcome: 02.03.03 Describe how buffers are important to living organisms. Mader - Chapter 02 #70 Section: 02.03 Topic: Chemistry |
| 71. | Briefly describe the major functions of lipids in the human body. Answers will vary but should include the following information. Fats and oils function as energy storage molecules. Phospholipids form the cell membrane and inner compartments of the cell. Steroids include the sex hormones. |
| Blooms Level: 6. Create Learning Outcome: 02.06.02 Identify the functions lipids play in our bodies. Mader - Chapter 02 #71 Section: 02.06 Topic: Chemistry |
| 72. | During the formation of a polymer, two monomers are joined by the removal of a _______ & _______.
Polymers are formed when H & OH are removed from the monomers during a dehydration reaction. COH, SH, NHH & COOH are all functional groups. |
| Blooms Level: 2. Understand Learning Outcome: 02.04.03 Recognize how monomers are joined to form polymers. Mader - Chapter 02 #72 Section: 02.04 Topic: Chemistry |
| 73. | Cholesterol is a component of cell membranes and is an example of which type of lipid?
Due to the structure of cholesterol it is classified as a steroid within the body. |
| Blooms Level: 2. Understand Learning Outcome: 02.06.02 Identify the functions lipids play in our bodies. Mader - Chapter 02 #73 Section: 02.06 Topic: Chemistry |
| 74. | What type of reaction is necessary to produce a dipeptide from individual amino acids?
Two amino acids are joined during a dehydration reaction to form a dipeptide. Hydrolysis reactions will break apart a dipeptide into individual amino acids. Denaturation is the change in shape of a protein. Dipeptides are formed from amino acids. |
| Blooms Level: 2. Understand Learning Outcome: 02.07.02 Explain how a polypeptide is constructed from amino acids. Mader - Chapter 02 #74 Section: 02.07 Topic: Chemistry |
| 75. | Determine what would happen to an individual's proteins if they developed a fever of 105o F for several days.
An increase in body temperature for several days would cause the proteins to denature. Once they denatured they would not be able to function correctly. Proteins would not increase in their ability to function due to an increase in body temperature. |
| Blooms Level: 5. Evaluate Learning Outcome: 02.07.01 Describe the functions of proteins in cells. Mader - Chapter 02 #75 Section: 02.07 Topic: Chemistry |
| 76. | Which group of lipids will contain hydrophilic heads that face outwards and hydrophobic tails that face inwards that will form a barrier?
Phospholipids have hydrophilic heads and hydrophobic tails that will form a barrier. Steroids have a backbone of four fused carbon rings. Triglycerides is formed from three fatty acids and a glycerol. Saturated acids and trans-fatty acids are both made from hydrocarbon chains with an acidic group on the end. |
| Blooms Level: 2. Understand Learning Outcome: 02.06.01 Compare the structures of fats, phospholipids, and steroids. Mader - Chapter 02 #76 Section: 02.06 Topic: Chemistry |
| 77. | Which nutrient source is the easiest one for humans to breakdown and form ATP?
Glucose is the easiest substance to breakdown into ATP. Proteins, cellulose, phospholipids and chitin are not easily broken down into ATP. |
| Blooms Level: 2. Understand Learning Outcome: 02.08.02 Explain the role of ATP in the cell. Mader - Chapter 02 #77 Section: 02.08 Topic: Chemistry |
| 78. | Which of the following cellular functions does not require ATP?
All of these are functions of ATP within the cell. |
| Blooms Level: 2. Understand Learning Outcome: 02.08.02 Explain the role of ATP in the cell. Mader - Chapter 02 #78 Section: 02.08 Topic: Chemistry |
| 79. | Briefly describe how ATP is broken down and turned into ADP. Answers will vary but should include the following: The last two phosphate bonds in ATP are unstable and easily broken. The terminal phosphate bond is hydrolyzed leaving ADP and an inorganic phosphate. Energy is released when the phosphate is broken off. |
| Blooms Level: 6. Create Learning Outcome: 02.08.02 Explain the role of ATP in the cell. Mader - Chapter 02 #79 Section: 02.08 Topic: Chemistry |
| 80. | What type of bond will connect the amino acids in a protein?
A peptide bond will connect the amino acids together to form a protein. The atoms associate with a peptide bond unevenly because the oxygen is more electronegative than nitrogen. |
| Blooms Level: 1. Remember Learning Outcome: 02.07.02 Explain how a polypeptide is constructed from amino acids. Mader - Chapter 02 #80 Section: 02.07 Topic: Chemistry |
| 81. | Determine which of the following components are required for a molecule to be classified as organic.
Organic molecules will have carbon, hydrogen, four electrons in the outer orbital, and a functional group attached to it. Sodium, chloride and oxygen are not necessary for organic molecules. Protons are not found in the outer orbitals. |
| Blooms Level: 5. Evaluate Learning Outcome: 02.04.01 Compare inorganic molecules to organic molecules. Mader - Chapter 02 #81 Section: 02.04 Topic: Chemistry |
ch2 Summary
| Category | # of Questions |
| Blooms Level: 1. Remember | 38 |
| Blooms Level: 2. Understand | 22 |
| Blooms Level: 3. Apply | 9 |
| Blooms Level: 4. Analyze | 6 |
| Blooms Level: 5. Evaluate | 4 |
| Blooms Level: 6. Create | 2 |
| Figure: 02.24 | 1 |
| Learning Outcome: 02.01.01 Describe how protons, neutrons, and electrons relate to atomic structure. | 3 |
| Learning Outcome: 02.01.02 Use the periodic table to determine relationships among atomic number and mass number. | 3 |
| Learning Outcome: 02.01.03 Describe how variations in an atomic nucleus account for its physical properties. | 3 |
| Learning Outcome: 02.01.04 Identify the beneficial and harmful uses of radiation. | 3 |
| Learning Outcome: 02.02.01 Describe how elements are combined into molecules and compounds. | 3 |
| Learning Outcome: 02.02.02 List the different types of bonds that occur between elements. | 7 |
| Learning Outcome: 02.02.03 Compare the relative strengths of ionic, covalent, and hydrogen bonds. | 3 |
| Learning Outcome: 02.03.01 Evaluate which properties of water are important for biological life. | 6 |
| Learning Outcome: 02.03.02 Identify common acidic and basic substances. | 6 |
| Learning Outcome: 02.03.03 Describe how buffers are important to living organisms. | 3 |
| Learning Outcome: 02.04.01 Compare inorganic molecules to organic molecules. | 4 |
| Learning Outcome: 02.04.02 Identify the role of a functional group. | 3 |
| Learning Outcome: 02.04.03 Recognize how monomers are joined to form polymers. | 3 |
| Learning Outcome: 02.05.01 Identify the structural components of a carbohydrate. | 2 |
| Learning Outcome: 02.05.02 List several examples of important monosaccharides and polysaccharides. | 4 |
| Learning Outcome: 02.06.01 Compare the structures of fats, phospholipids, and steroids. | 4 |
| Learning Outcome: 02.06.02 Identify the functions lipids play in our bodies. | 3 |
| Learning Outcome: 02.07.01 Describe the functions of proteins in cells. | 4 |
| Learning Outcome: 02.07.02 Explain how a polypeptide is constructed from amino acids. | 3 |
| Learning Outcome: 02.07.03 Compare the four levels of protein structure. | 6 |
| Learning Outcome: 02.08.01 Compare the structure and function of DNA and RNA. | 4 |
| Learning Outcome: 02.08.02 Explain the role of ATP in the cell. | 3 |
| Mader - Chapter 02 | 81 |
| Section: 02.01 | 12 |
| Section: 02.02 | 13 |
| Section: 02.03 | 15 |
| Section: 02.04 | 10 |
| Section: 02.05 | 6 |
| Section: 02.06 | 7 |
| Section: 02.07 | 12 |
| Section: 02.08 | 7 |
| Topic: Chemistry | 81 |
Instructor’s Manual
to accompany
Inquiry into Life
Fourteenth Edition
Sylvia Mader

PREFACE
Inquiry into Life is a textbook for college freshman biology courses and covers the whole field of basic biology. The textbook emphasizes the application of biology to human life and the relationship of humans to other organisms. Students should discover basic biology principles and also understand that biology concepts are relevant to everyday living. This Instructor’s Manual is designed to assist you as you plan and prepare for classes using Inquiry into Life.
ORGANIZATION OF THE INSTRUCTOR’S MANUAL
Chapter Contents
Learning Outcomes
Learning outcomes presented in the Instructor’s Manual give the instructor a quick overview of the major themes covered in the chapter.
Lecture Outline
Each chapter in Inquiry into Life begins with a “Chapter Outline.” This outline is expanded with annotations in the Instructor’s Manual and has several functions. An instructor can use it for his/her lecture notes since it contains the important concepts from the textbook and is cross-referenced to topic headings in the textbook. The outline can be made into Microsoft Office PowerPoint slides so the students can follow along with the lecture. Or an instructor may reproduce the extended outline to give to students before or after the lecture on that particular chapter. The organization of lecture concepts is a critical decision made by each instructor. A disorganized presentation is more difficult for students to follow and the distinct concepts may not build to an overall understanding. The selection of examples to illustrate concepts, and the selection of which concepts to represent and what order they should be presented, are critical factors in good teaching. The organization given here reflects the organization and voice of the textbook author, Dr. Sylvia Mader.
Connections & DVD Resources
Each chapter in the Instructor’s Manual lists web addresses for online connections that are useful for finding further information and examples for teaching. DVD resources provide visual learning assistance for students. Producers have usually indicated their materials are appropriate for adult and college levels, and titles are listed without any recommendation as to the content, quality, or year of production. Further information can be obtained by contacting the distributors.
Lecture Enrichment Ideas
This section suggests unique presentation and lecture strategies and methods to involve students in and out of the classroom. These topics and projects are not all-inclusive, but may help an instructor try something new and increase the interest of students. Lists of suggested term paper topics are provided so a teacher doesn’t have to “reinvent the wheel.”
Essay Questions with Answers
Each section contains several essay questions with answers for inclusion on student performance evaluations.
DISTRIBUTORS OF DVDS
Amazon.com
http://www.amazon.com/
Ambrose Video Publishing, Inc.
145 W. 45th Street, Suite 1115
New York, NY 10036
1-800-526-4663
http://www.documentary-video.com/order.cfm
Annenberg Media
PO Box 55742
Indianapolis, IN 46205-0742
1-800-532-7637
http://www.learner.org/index.html
Carolina Biological Supply
2700 York Road
Burlington, NC 27215-3398
1-800-334-5551
http://www.carolina.com/home.do
Educational Video Network
1401 19th Street
Huntsville, TX 77340
1-800-762-0060
https://www.evndirect.com/index.php
Films Media Group
Films for the Humanities and Sciences
200 American Metro Blvd., Suite 124
Hamilton, NJ 08619
1-800-257-5126
http://ffh.films.com
Garland Science
Mortimer House
37-41 Mortimer Street
London, W1T 3JH
GB 365 4626 36
http://www.garlandscience.com/textbooks/0815342233.asp
Great Pacific Media
P.O. Box 26243
Colorado Springs, CO 80936
1-800-325-1956
http://www.greatpacificmedia.com/product_p/Glycolysis_dvd.htm
National Geographic Catalog/Online
777 South State Road 7
Margate, FL 33068
1-888-225-5647
http://shop.nationalgeographic.com/
UNIT 1: CELL BIOLOGY
CHAPTER 1: THE STUDY OF LIFE
LEARNING OUTCOMES
1.1 The Characteristics of Life
1. Identify the basic characteristics of life.
2. Distinguish between the levels of biological organization.
1.2 The Classification of Organisms
1. Describe how living things are classified.
2. Distiniguish between the three domains of life.
1.3 The Organization of the Biosphere
1. Distinguish among populations, communities, ecosystems, and the biosphere.
2. List ways in which humans influence ecosystems.
1.4 The Process of Science
1. Distinguish between a theory and a hypothesis.
2. Identify the components of the scientific method.
3. Analyze a scientific experiment and identify the hypothesis, experiment, control groups, and conclusions.
1.5 Science and Social Responsibility
1. Identify the costs and benefits of technology.
2. Explain what could happen to the human population if we stopped using technology.
LECTURE OUTLINE
1.1 The Characteristics of Life
The diversity of life seems overwhelming, and yet all living things have certain characteristics in common.
Organisms Are Organized
Organisms can be organized in a hierarchy of levels. A cell is the smallest unit of life.
Organisms Acquire Materials and Energy
Organisms need an outside source of materials and energy to maintain their organization or carry on life’s other activities.
Organisms Reproduce
Life comes only from life.
Organisms Respond to Stimuli
Organisms respond to external stimuli, often by moving toward or away from a stimulus.
Organisms Are Homeostatic
Homeostasis means “staying the same.” The internal environment of an organism stays relatively constant.
Organisms Grow and Develop
Growth, recognized by an increase in the size of an organism and often in the number of cells, is a part of development.
Organisms Have the Capacity to Adapt
Natural selection results when adaptations, which are certain features that make organisms better suited to an environment, allow those individuals of a species to be able to reproduce and pass on those characteristics. Evolution explains both the unity and diversity of life.
1.2 The Classification of Organisms
Because life is so diverse, it is helpful to have a classification system to group organisms based upon their similarities.
Domains
Domains are the largest classification category. There are three domains: Archaea, Bacteria, and Eukarya. Archaea and Bacteria are unicellular prokaryotes, which lack the membrane-bounded nucleus found in the cells of eukaryotes, which make up the Eukarya.
Kingdoms
Systematists are in the process of deciding how to categorize archaea and bacteria into kingdoms. The eukaryotes are currently classified into four kingdoms: protists, fungi, plants, and animals.
Other Categories
The other classification categories are phylum, class, order, family, genus, and species.
Scientific Names
Taxonomy is the assignment of a binomial, or two-part name, to each species, where the first word is the genus to which the species belongs and the second word is the species. Scientific names are in a common language—Latin.
1.3 The Organization of the Biosphere
The organization of life extends beyond the individual to the population, community, ecosystem, and finally the biosphere, which is the zone of air, land, and water on Earth where living organisms are found.
The Human Species
The human species tends to modify existing ecosystems for its own purposes. Humans depend on healthy ecosystems for food, medicines, and various raw materials.
Biodiversity
Biodiversity encompasses the total number of species, the variability of their genes, and the ecosystems in which they live.
1.4 The Process of Science
Biology is the scientific study of life.
Observation
Natural phenomena can be understood more fully by observing and studying them.
Hypothesis
After making observations and gathering knowledge about a phenomenon, a scientist uses inductive reasoning to come up with a hypothesis, a tentative explanation for the natural event.
Experiment/Further Observations
Testing a hypothesis involves either conducting an experiment or making further observations.
Data
The results of an experiment are referred to as the data. Data should be observable and objective, rather than subjective or based on opinion.
Conclusion
Scientists must analyze the data to reach a conclusion to determine if the hypothesis can be supported or not.
Scientific Theory
The ultimate goal of science is to understand the natural world in terms of scientific theories, concepts that join together well-supported and related hypotheses.
A Controlled Study
Most investigators perform controlled studies in which the experimental group receives a treatment and the control group receives no treatment.
The Experiment
The pigeon pea plant is a legume with a high rate of atmospheric nitrogen conversion. A hypothesis was outlined involving winter wheat and nitrogen fertilizer.
The Results
The results lead to the conclusion that the hypothesis is not supported.
Continuing the Experiment
The researchers modified the hypothesis to involve sustained effects.
The Results
The results lead to the conclusion that the hypothesis was supported.
Ecological Importance of This Study
This study showed that the use of a legume improved the soil to produce a better yield than the use of a nitrogen fertilizer over the long haul.
1.5 Science and Social Responsibility
The application of scientific knowledge for a practical purpose is called technology. Most technologies have benefits but also drawbacks. Making value judgments is not a part of science. Ethical and moral decisions about technology must be made by all people.
CONNECTIONS
The Committee for Skeptical Inquiry (CSI) promote scientific inquiry, critical investigation, and the use of reason in examining controversial and extraordinary claims. CSI publishes the Skeptical Inquirer (6/year); highly useful examples of applying science attitudes to psychic claims, etc. Available from Skeptical Inquirer, http://www.csicop.org
The National Academies of Science provides a guide to research issues in the life sciences at http://nationalacademies.org/
The Skeptics Society’s mission is to engage leading experts in investigating the paranormal, fringe science, pseudoscience, and extraordinary claims of all kinds; publishes The Skeptic available from http://www.skeptic.com
Sigma Xi, the Scientific Research Society discusses science attitudes and fraud in its publication American Scientist available at a small cost, http://www.sigmaxi.org
Virtual Institute of Cryptozoology is the website for the International Society of Cryptozoology (ISC) dedicated to investigating reports of rarely seen animals at http://perso.wanadoo.fr/cryptozoo/method.htm
DVD RESOURCES
Biotechnology in the 21st Century, ISBN 978-1-60825-071-4, Films for the Humanities and Science, http://ffh.films.com
Introduction to Designing Experiments, ISBN 978-1-4213-5431-6, Films for the Humanities and Science, http://ffh.films.com
Scientific Method, ISBN 978-1-4213-7403-1, Films for the Humanities and Science, http://ffh.films.com
LECTURE ENRICHMENT IDEAS
1. Describe the many properties of quartz crystals: that crystals “grow” over time, little crystals are “reproduced” at angles off the sides of parent crystals, the crystal possesses a predictable angular “structure” that is not like the chaotic environment, and sheets of quartz even show a response to environmental stimuli—they convert light to current in a piezoelectric effect! With so many features of life, why isn’t quartz considered living?
2. We are sending additional robot spacecraft to Mars to further investigate the possibility of life on Mars. In the past, we have looked for properties that life expresses on earth: respiration, growth, movement, etc. Discuss whether the model of Earth’s properties of life is the only model possible.
3. Buy a recent tabloid newspaper from the newsstands featuring a pseudoscience topic (students
readily recognize these from the grocery checkout stands). Read one brief account of a
particularly preposterous assertion and ask what is necessary for a scientist to believe this, what
internal contradictions belie its claims, and what tests would be necessary to provide it with
scientific legitimacy, etc.
4. Display an assortment of screws, bolts, nails, brads, staples and other fasteners. Ask students
to “classify” them in groups for easier display in a store, etc. On what basis do they group screws
and bolts, tacks and nails, etc. (common structures, threading, production methods,
functions, etc.)? The basis for biological classification is commonly phylogenetic origin but
likewise uses common structures and functions.
5. Have students propose various scientific experiments based on their observations. Discuss how to control the variables, how to obtain objective data, how to interpret results, and so on.
6. Have students read the Science in Your Life-Health “How Safe is Your Cell Phone?” before coming to class. Discuss the answers to the discussion questions at the end of the reading.
ESSAY QUESTIONS WITH ANSWERS
1. By itself, a virus cannot reproduce, grow and develop, respond to stimuli, take in material and energy from the environment, nor show any adaptation. However, it is organized with a protein coat that surrounds hereditary material and once inside another living cell the virus takes over the host’s metabolic machinery in order to grow, develop, and reproduce new virus particles. Therefore, is the virus a living or dead organism?
Answer: Scientists do not have a unanimous answer to this question because viruses have characteristics of being both alive and dead. Because viruses are unable to carry out metabolic processes themselves, some scientists prefer to consider viruses activated and inactivated.
2. Explain what is scientifically wrong with the following assertions:
a. I clang bells each day and there are no tigers around my house; therefore clanging bells drives away tigers.
Answer: There is no test with tigers present; therefore there is no cause-and-effect established.
b. If water dousing, homeopathic cures, and so on work for just me but not for anyone else, it is still science.
Answer: Science is not personal; results must be repeatable.
3. Why does evolution explain both the unity and the diversity of life?
Answer: All organisms share the same basic characteristics of life because we all share a common ancestor (unity). During the past 4 billion years, Earth’s environment has changed drastically, and the diversity of life has been shaped by the evolutionary responses of organisms to these changes (diversity).
UNIT 1: CELL BIOLOGY
CHAPTER 2: THE MOLECULES OF CELLS
LEARNING OUTCOMES
2.1 Basic Chemistry 1. Define how protons, neutrons, and electrons relate to atomic structure.
2. Use the periodic table to determine relationships among atomic number and mass number.
3. Describe how variations in an atomic nucleus account for its physical properties.
4. Identify the beneficial and harmful uses of radiation.
2.2 Molecules and Compounds
1. Describe how elements are combined into molecules and compounds.
2. List the different types of bonds that occur between elements.
3. Compare the relative strengths of ionic, covalent, and hydrogen bonds.
2.3 Chemistry of Water
1. Evaluate which properties of water are important for biological life.
2. Identify common acidic and basic substances.
3. Describe how buffers are important to living organisms.
2.4 Organic Molecules
1. Compare inorganic molecules to organic molecules.
2. Identify the role of a functional group.
3. Recognize how monomers are joined to form polymers.
2.5 Carbohydrates
1. Identify the structural components of a carbohydrate.
2. List several examples of important monosaccharides and polysaccharides.
2.6 Lipids
1. Compare the structures of fats, phospholipids, and steroids.
2. Identify the functions lipids play in our bodies.
2.7 Proteins
1. Describe the functions of proteins in cells.
2. Explain how a polypeptide is constructed from amino acids.
3. Compare the four levels of protein structure.
2.8 Nucleic Acids
1. Compare the structure and function of DNA and RNA.
2. Explain the role of ATP in the cell.
LECTURE OUTLINE
2.1 Basic Chemistry
Matter refers to anything that takes up space and has mass. All matter, both nonliving and living, is composed of certain basic substances called elements.
Atomic Structure
Elements consist of tiny particles called atoms, which are the smallest part of an element that displays the properties of that element. Atoms are made up of positively charged protons, uncharged neutrons, and negatively charged electrons. All atoms of an element have the same number of protons. This number is called the atomic number. The mass number is the sum of an atom’s protons and neutrons.
The Periodic Table
The periodic table was constructed as a way to group the elements according to certain chemical and physical characteristics.
Isotopes
Isotopes are atoms of the same element that differ in their number of neutrons, therefore isotopes have the same number of protons, but their mass number is different.
Low Levels of Radiation
The chemical behavior of a radioactive isotope is essentially the same as that of the stable isotopes of an element so you can use small amounts of radioactive isotopes as tracers to detect molecular changes.
High Levels of Radiation
Radioactive substances in the environment can harm cells, damage DNA, and cause cancer.
Electrons
In an electrically neutral atom, the positive charges of the protons in the nucleus are balanced by the negative charges of electrons moving outside the nucleus in orbitals. The number of electrons in the outer orbital determines whether an atom reacts with other atoms.
2.2 Molecules and Compounds
A molecule is formed when two or more atoms bond together. When the atoms of two or more different elements bond together, the product is called a compound.
Ionic Bonding
Ions form when electrons are transferred from one atom to another. Ionic compounds are held together by an attraction between negatively and positively charged ions called an ionic bond.
Covalent Bonding
A covalent bond results when two atoms share electrons in such a way that each atom has a complete outer orbital.
Shape of Molecules
Molecules have a three-dimensional shape that often determines their biological function. The shapes of molecules are necessary to the structural and functional role they play in living things.
Nonpolar and Polar Covalent Bonds
When the sharing of electrons between two atoms is fairly equal, the covalent bond is said to be nonpolar. The unequal sharing of electrons in a covalent bond results in a slightly negative charge and a slightly positive charge, resulting in a polar covalent bond.
Hydrogen Bonding
Polarity within a water molecule causes the hydrogen atoms in one molecule to be attracted to the oxygen atoms in other water molecules, forming a hydrogen bond.
2.3 Chemistry of Water
The unique properties of water make it essential to the existence of life.
Properties of Water
The many hydrogen bonds that link water molecules help water absorb heat without a great change in temperature. Water has a high heat of vaporization because hydrogen bonds must be broken before water boils and changes to a vaporized state. Water is a solvent; due to its polarity, it facilitates chemical reactions inside and outside living organisms. Water molecules are cohesive and adhesive, it flows freely, but its molecules do not separate, and it is able to adhere to polar surfaces. The strong hydrogen bonds between water molecules results in high surface tension. Ice is less dense than liquid water, and therefore ice floats.
Acids and Bases
When water ionizes, it releases an equal number of hydrogen ions and hydroxide ions.
Acid Solutions (High H+ Concentrations)
Acids are substances that release hydrogen ions (H+) when they dissociate in water.
Basic Solutions (Low H+ Concentration)
Bases are substances that either take up hydrogen ions (H+) or release hydroxide ions (OH-).
pH Scale
The pH scale is used to indicate the acidity or basicity (alkalinity) of solutions. The pH scale ranges from 0 to 14.
Buffers and pH
A buffer is a substance that keeps pH within normal limits. In animals, the pH of body fluids is maintained within a narrow range, or else health suffers.
2.4 Organic Molecules
Organic molecules always contain carbon (C) and hydrogen (H). A functional group is a specific combination of bonded atoms that always react in the same way. A monomer is a simple organic molecule that exists individually or can link with other monomers to form a polymer. Dehydration and hydrolysis reactions join monomers and degrade polymers.
2.5 Carbohydrates
Carbohydrates function for quick fuel and short-term energy storage in all organisms. Carbohydrate molecules are characterized by the presence of the atomic grouping H—C—OH.
Simple Carbohydrates
If the number of carbon atoms in a molecule is low, then the carbohydrate is a simple sugar, or monosaccharide. Glucose is a sugar found in our blood. A disaccharide contains two monosaccharides that have joined during a dehydration reaction.
Polysaccharides
Long polymers such as starch, glycogen, and cellulose are polysaccharides that contain many glucose subunits.
Starch and Glycogen
Starch and glycogen are large storage forms of glucose in plants and animals.
Cellulose
The polysaccharide cellulose is found in plant cell walls, which accounts for the strong nature of these walls.
No comments:
Post a Comment